Ventra Labs’ Comprehensive Testing Portfolio
Test menu settings can be edited in the left-side menu.
Core Laboratory Tests
Routine – 24-hour turnaround time
Stat – 4-hour turnaround
AU 480 – Routine Blood Chemistry DxH 690 – Hematology / Sysmex – Coagulation
⦁ Complete Blood Count (CBC) w/Differential
⦁ Erythrocyte Sedimentation Rate (ESR)
⦁ Hemoglobin A1C
⦁ Hemoglobin & Hematocrit (H&H)
⦁ Reticulocytes
⦁ PT, PTT, INR
DXI 600 – Immunoassay/Hormones
⦁ Prostaglandin
⦁ Prolactin
⦁ Prostate-specific Antigen (PSA)
⦁ Parathyroid Hormone (PTH)
⦁ SHBG
⦁ Testosterone
⦁ Thyroid Peroxidase (Ab)
⦁ Thyroxine (T4)
⦁ B12
⦁ Vitamin D
⦁ Human Chorionic Gonadotropin (HCG)
⦁ Cortisol
⦁ DHEA-S
⦁ Estradiol
⦁ Folate
⦁ Follicle-Stimulating Hormone (FSH)
⦁ Levetiracetam
Chemistry
- Albumin
- Alkaline Phosphatase
- Alanine aminotransferase (ALT)
- Aspartate phosphatase (AST)
- Amylase
- APO A1
- APO B
- Basic Metabolic Panel (BMP)
- Direct Bilirubin
- Total Bilirubin
- Blood Urea Nitrogen (BUN)
- Complete Metabolic Panel (CMP)
- Carbon Dioxide (CO2)
- Creatine Phosphokinase (CPK)
- Cholesterol
- Creatinine
- Cystatin C (CYSC)
- High-Sensitivity CRP
- Sodium, Magnesium, Calcium, Chloride, Potassium, Phosphorous
- Ferritin
- Glucose
- Gamma-glutamyl transferase (CGT)
- HDL
- LDL
- Iron
- LDH
- Lipase
- Lipoprotein A
- Lithium
- Luteinizing Hormone (LH)
- Rheumatoid Factor (RF)
- Total Iron-Binding Capacity (TIBC)
- Total Protein
- Troponin
- Triglycerides
- Uric Acid
- Urinalysis
- Valproic Acid
- Microalbumin
Molecular (RT-PCR) Panels
24-48 hour turnaround time
qPLEX UTI + ABR Panel
UTI Pathogens
- Acinetobacter baumannii
- Enterococcus faecalis
- Escherichia coli
- Staphylococcus aureus
- Enterobacter cloacae
- Klebsiella oxytoca
- Klebsiella pneumoniae
- Pseudomonas aeruginosa
- Citrobacter freundii
- Morganella morganii
- Proteus mirabilis
- Proteus vulgaris
- Enterococcus faecium
- Klebsiella aerogenes
- Candida lusitaniae
- Candida parapsilosis
- Mycoplasma hominis
- Ureaplasma urealyticum
- Providencia stuartii
- Serratia marcescens
- Staphylococcus saprophyt.
- Streptococcus agalactiae
- Candida albicans
- Candida glabrata
- Candida krusei
- Candida tropicalis
- Candida auris
Antibiotic Resistance Markers
- Amp C Resistance Markers – ampC
- Methicillin Resistance Markers – mecA, femA
- Vancomycin Resistance Markers – vanA1, vanA2, vanB,
- Quinolone and Fluoroquinolone Resistance – QnrA, QnrB
- Carbapenem Resistance Markers – NDM, KPC, OXA-48, VIM, IMP-7
- ESBL Resistance Markers – SHV, TEM, CTX-M group 1, CTX-M group 2
- Macrolide Resistance Markers – mefA, EmA, EmB
qPLEX Wound + ABR Panel
Wound Pathogens
- Bacteriodes fragilis
- Citrobacter freundii
- Enterobacter cloacae
- Morganella morganii
- Staphylococcus epidermidis
- Staphylococcus aureus
- Acinetobacter baumannii
- Enterococcus faecalis
- E. coli
- Pseudomonas aeruginosa RESISTANCE MARKERS
- Klebsiella oxytoca
- Klebsiella pneumoniae
- Proteus mirabilis
- Proteus vulgaris
- Enterobacter aerogenes
- Enterobacter faecium
- Kingella kingae
- Clostridium novyi
- Clostridium septicum
- Clostridium perfringens
- Group A Strep
- Group B Strep
- Group C & G Strep
Antibiotic Resistance Markers
- Amp C Resistance Markers – ampC
- Methicillin Resistance Markers – mecA, femA
- Vancomycin Resistance Markers – vanA1, vanA2, vanB,
- Quinolone and Fluoroquinolone Resistance – QnrA, QnrB
- Carbapenem Resistance Markers – NDM, KPC, OXA-48, VIM, IMP-7
- ESBL Resistance Markers – SHV, TEM, CTX-M group 1, CTX-M group 2
- Macrolide Resistance Markers – mefA, EmA, EmB
VIASURE RSV/SARS-CoV-2/Flu A&B Panel
The VIASURE SARS-CoV-2, Flu & RSV Real Time PCR Test is designed for the identification of SARS-CoV-2, Influenza A/B (Flu A/B), and/or Human Respiratory Syncytial Virus A/B (RSV A/B) in respiratory specimens from patients exhibiting signs and symptoms of respiratory infection.
ATILA Biosystems Nail Fungus Panel
Our nucleic acid-based amplification test (NAAT) utilizes semi-quantitative real-time polymerase chain reaction (RT- PCR) technology to help clinicians identify the fungal pathogens found in nail tissue, enabling doctors to begin targeted therapy within days.
Our RT-PCR assay can accurately identify unique nucleic acid segments of each pathologic fungi or bacteria at genus and some at species level. Our test is performed using the patient’s nail clippings, and multiple pathogens can be detected in one test.
VIASURE Gastrointestinal Pathogens Panel IV
The VIASURE Gastrointestinal Panel IV Real Time PCR Detection Kit is designed for the specific identification and differentiation of Clostridium difficile Toxin A and B; Salmonella, Campylobacter and/or Yersinia enterocolitica; Campylobacter coli, Campylobacter lari and/or Campylobacter jejuni; and/or Enterohemorrhagic Escherichia coli (EHEC), Shiga toxin-roducing Escherichia coli (STEC), Enteropathogenic Escherichia coli (EPEC) and/or Enteroinvasive Escherichia coli (EIEC)/Shigella in human stool samples from patients with signs and symptoms of gastrointestinal infection.
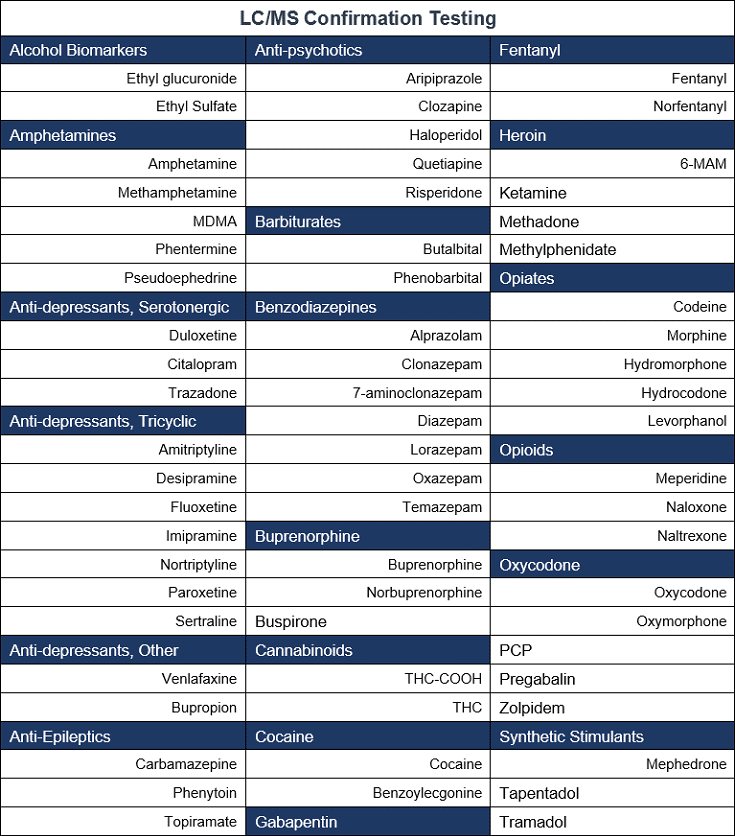
LC/MS Toxicology
48-72 hour turnaround time
Oral Fluid Confirmation Testing/Urine Confirmation Testing